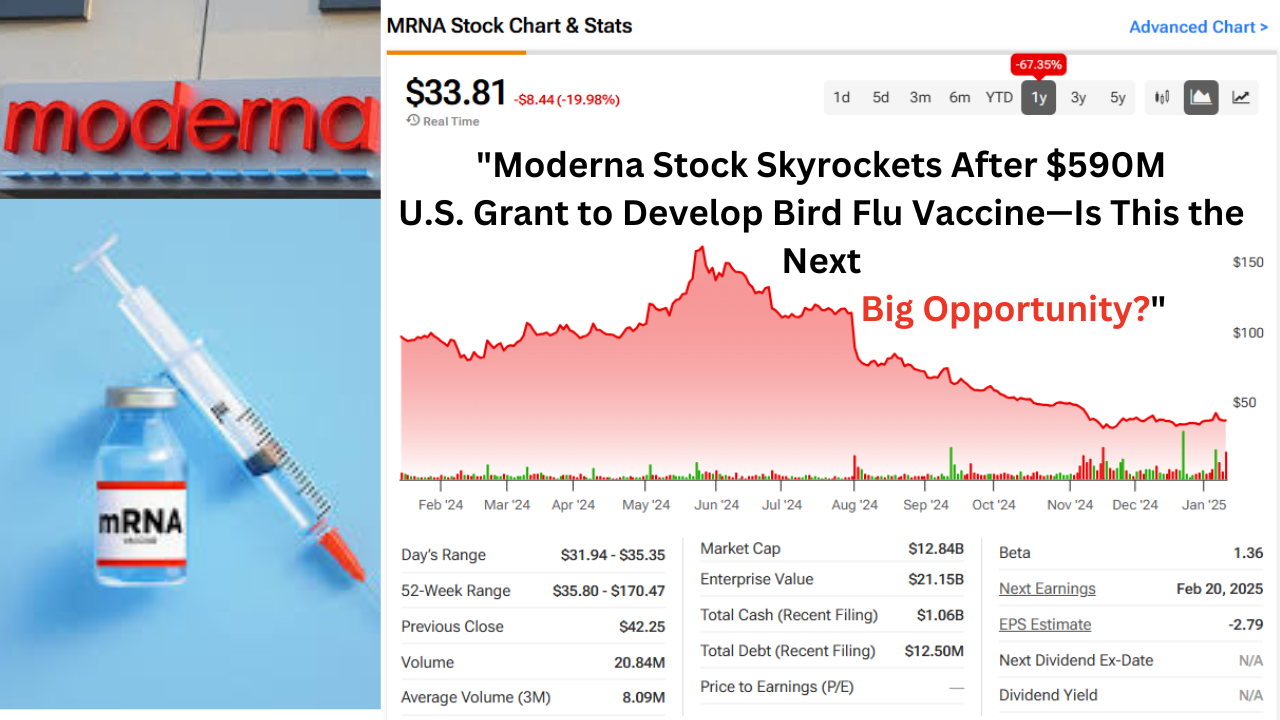
Moderna, the biotech company that gained global recognition for its COVID-19 vaccine, is back in the spotlight. This time, the company is receiving a substantial boost from the U.S. government to tackle another health threat: bird flu. The U.S. Department of Health and Human Services (HHS) has awarded Moderna a $590 million grant to speed up the development of a vaccine aimed at preventing future pandemics caused by avian influenza.

What’s Happening with Moderna’s Bird Flu Vaccine?
Moderna’s new vaccine candidate, called mRNA-1018, is being developed using the same mRNA technology that powered its successful COVID-19 vaccine. The company is focusing on targeting the H5 and H7 strains of the bird flu virus, which have been causing growing concerns worldwide. These strains have been found in wild birds and have led to outbreaks in poultry, with a few recent human cases reported in U.S. dairy and poultry workers.
After positive results from early-stage trials, Moderna is now advancing mRNA-1018 into a Phase 3 study. This development could help Moderna respond faster to future flu outbreaks and pandemics, much like it did with COVID-19.
Why Is This Important?
While bird flu cases in humans are still relatively rare, the World Health Organization (WHO) and U.S. health officials have been monitoring the situation closely. With the bird flu spreading rapidly in wildlife and poultry, the U.S. government is working to ensure that the country is prepared for potential future outbreaks.
In fact, some experts believe the risk of bird flu becoming a bigger health threat is increasing. This is why the U.S. government is investing heavily in research and development, as well as the creation of effective vaccines that could be quickly distributed if needed.
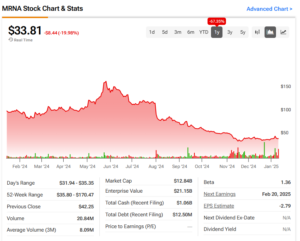
Moderna’s Stock Reaction
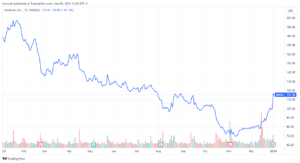
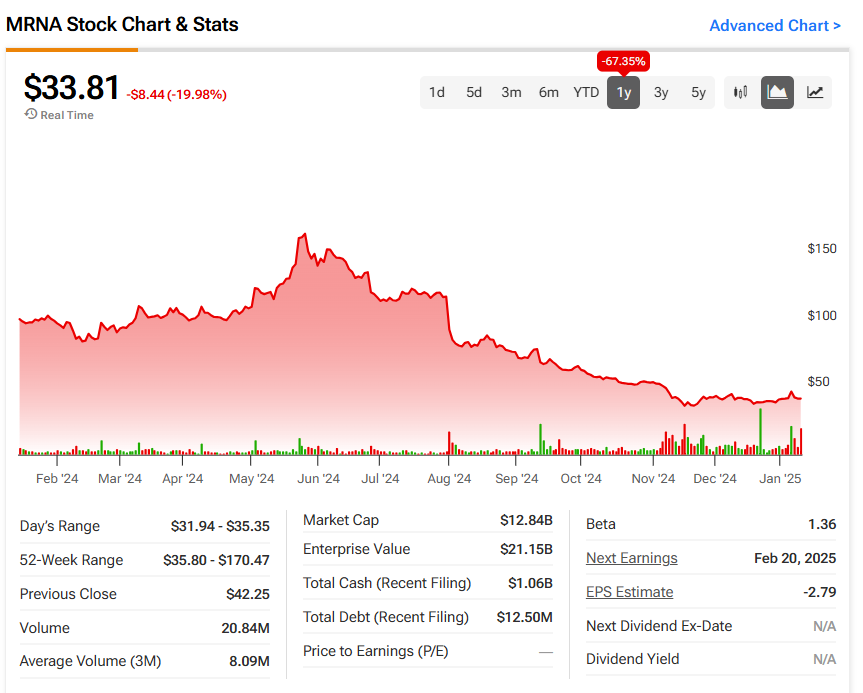
Following the announcement of the $590 million grant, Moderna’s stock surged by nearly 5%. However, it’s important to note that the company’s shares are still down by around 65% compared to the same time last year, mainly due to falling sales of its COVID-19 vaccine. Despite the dip, the company’s new ventures in pandemic prevention, including the bird flu vaccine, are giving investors new hope for its future growth.
The Bigger Picture: What’s Next?
Moderna’s decision to focus on mRNA vaccines for multiple diseases marks a significant step in the fight against future pandemics. The success of its COVID-19 vaccine showed the world how quickly and effectively mRNA technology can be adapted to fight emerging viruses. With this new grant from the U.S. government, Moderna is positioning itself as a key player in the global effort to prevent and combat flu pandemics.
The development of an effective bird flu vaccine could also help protect global food supplies. As bird flu outbreaks affect poultry farms, they can lead to disruptions in egg production, which has been a growing concern in recent months. For example, Cal-Maine Foods, the largest egg producer in the U.S., recently stated that egg prices are continuing to rise because of limited production due to bird flu.
What’s the Takeaway for Investors?
While Moderna’s COVID-19 vaccine sales have slowed down, its pivot to developing mRNA vaccines for other diseases—like bird flu—could help revitalize its future prospects. The U.S. government’s backing is a clear sign that there’s serious investment in Moderna’s capabilities, and it could help boost both public health and the company’s bottom line in the long term.